Fracture of solid materials may arise as a result of the applied stress or purely as a result of the corrosive action of the environment. Referring only to stress induced fracture the two main mode are ductile and brittle. Most metals can withstand a large deformation, of the order of several percent, without failing. As the deformation increases past the yield point, microvoids are formed, as shown in Figure 1

Figure 1. Appearance of fracture surfaces from microvoid coalescence. From ASM Metals Handbook, 8th edn., Vol. 9, ASM International, Metals Park, Ohio (1994) with permission.
At any given instant, the fraction of microvoids, f, may be taken to define a "damage factor", increasing the true stress.
Fracture finally takes place when the microvoids coalesce and results in the dimpled appearance of the fracture surfaces. The dimples are equiaxed, in case of pure tension, or elongated when in the presence of shear, as is normally found near free surface where fracture follows from the slip along the planes of maximum shear stress. The ligaments between large clusters of microvoids may also tear, forming sharp ridges.
Metals are normally built up from crystals of atoms in the face-centered cubic, hexagonal close-packed or body-centered cubic structures. Of these, the face-centred cubic are the most ductile and always fail by the void coalescence mechanism after yield. Such metals include gold, copper and alloys such as brass and aluminium alloys.
Metals with the other crystal structures do not possess the easy dislocation motion of face-centered alloys and under low temperature or high strain rate they may fail by cleavage, e.g., separation occurs along well-defined crystal planes. The cleavage is not related to the total plastic deformation of the specimen.
From an engineering or macroscopic standpoint, brittle fracture is said to occur in a tensile specimen if no permanent deformation is observed in the two specimen halves, which may be fitted back together so that the appearance is that of the original specimen. The fracture mechanism may be cleavage, i.e., separation of planes within a grain, since cracks are associated with limited crystallographic slip and hence small strains, but the most characteristic mechanism is intergranular fracture. The fracture surfaces reveal the outline of the grains that have separated due to the presence of weak or brittle phases between them or to environmental effects such as stress corrosion or hydrogen embrittlement.
Brittle fracture is the main mode of failure of glass and ceramics. Fracture starts from a small flaw—typically a few microns—and develops into a fracture mirror before entering into a mist characterized by the presence of fine radial ridges. This is followed by a hackle, with longer, better defined radial lines. Finally, a major crack or cracks sweep through the rest of the surface.
Failure depends on the size of the starter flaw, on the applied stress and on the fracture toughness of the material, in accordance with the general expression

where Y is a dimensionless parameter depending on geometry, σ is the nominal applied stress, a the characteristic size of the flaw and Kc the fracture toughness. For a ceramic, Kc may be of the order of 5 MPa
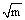 when the stress is normal to the plane of the flaw. In a steel, it may be as high as 150 MPa
when the stress is normal to the plane of the flaw. In a steel, it may be as high as 150 MPa
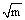 so that, for the same applied stress, the flaw needed to start a brittle fracture would be of the order of millimeters rather than of microns.
so that, for the same applied stress, the flaw needed to start a brittle fracture would be of the order of millimeters rather than of microns.
When a crack is present, a material that would otherwise fail in a ductile manner may fail without any prior plastic deformation provided that the crack size is sufficiently large or that external constraints inhibit the free plastic deformation. This occurs in large sections and in welded structures with a high level of residual stresses.
REFERENCES
Derby, B., Hills, D. A, and Ruiz, C. (1992) Materials for Engineering, Longman.
Fellows, J. A. Ed. (1974) Metals Handbook Vol. 9, Fractography and Atlas of Fractographs, Amer. Soc. Metals.
Использованная литература
- Derby, B., Hills, D. A, and Ruiz, C. (1992) Materials for Engineering, Longman.
- Fellows, J. A. Ed. (1974) Metals Handbook Vol. 9, Fractography and Atlas of Fractographs, Amer. Soc. Metals.