The concept of partial pressure applies, exactly, only to perfect gas mixtures, i.e., ones in which each component gas follows the equation of state  for all pressures and temperatures. Then:
for all pressures and temperatures. Then:

where 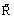 is the universal gas constant, T the absolute temperature and ni is the amount of component i present.
is the universal gas constant, T the absolute temperature and ni is the amount of component i present.
From this one can define a partial pressure of component i as,

or,

where  is the mole fraction of component i.
is the mole fraction of component i.
It is used, as an approximation, for real gases at low or moderate pressures. For permanent gases, the approximation is often acceptable up to atmospheric pressure, or even slightly above. For condensible gases, the approximation may be an order of magnitude worse.