When a gas in steady flow passes through a constriction, e.g., in an orifice or valve, it normally experiences a change in temperature. This is in part due to changes in kinetic energy, but there is another part contributed by the nonideality of the gas. If the upstream and downstream ducts are sufficiently large for kinetic energy to be negligible at these stations, upstream and downstream temperatures are measured far enough away from the disturbance created by the constriction and the system is adiabatic; the measured effect is due to the nonideality alone. From the first law of thermodynamics, such a process is isenthalpic and one can usefully define a Joule-Thomson coefficient as:
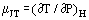
as a measure of the change in temperature which results from a drop in pressure across the constriction.
For most real gases at around ambient conditions, μ is positive—i.e., the temperature falls as it passes through the constriction. For hydrogen and helium, it is negative and the temperature increases. At higher temperatures, for most gases, μ falls and may even become negative, μ can also become negative through application of pressure, even at ambient temperature, but pressures in excess of 200 bar are normally necessary to achieve this.