Heat transfer is a physical process of spontaneous, irreversile heat transport from hotter (i.e., having a higher temperature) bodies or parts of bodies to cooler ones. It is also a field of science that is concerned with the study and quantitative description of heat transfer laws.
Heat transfer as a physical process of heat transport in a space with nonuniform temperature distribution is characterized by the heat flux  , W/m2, i.e., the quantity of heat passing per unit time through a unit of arbitrary surface along the normal to it. If the surface is represented by the surface or the wall of the body which is in a state of heat transfer with other bodies, e.g., between the wall and the fluid medium flowing over it, then we deal with the heat flux on the wall
, W/m2, i.e., the quantity of heat passing per unit time through a unit of arbitrary surface along the normal to it. If the surface is represented by the surface or the wall of the body which is in a state of heat transfer with other bodies, e.g., between the wall and the fluid medium flowing over it, then we deal with the heat flux on the wall 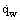 , which is one of the most important resultant characteristics of heat transfer. The quantity
, which is one of the most important resultant characteristics of heat transfer. The quantity  which depends on the character and scale of temperature nonuniformities in the system (in the wall-fluid system this scale is given as temperature difference ΔT = Tw — Tf, where Tf is the characteristic temperature of fluid, Tw the temperature of the wall, on the geometrical characteristics of the system, on the characteristics of fluid flow and by the physical properties of the bodies and media participating in heat transfer.
which depends on the character and scale of temperature nonuniformities in the system (in the wall-fluid system this scale is given as temperature difference ΔT = Tw — Tf, where Tf is the characteristic temperature of fluid, Tw the temperature of the wall, on the geometrical characteristics of the system, on the characteristics of fluid flow and by the physical properties of the bodies and media participating in heat transfer.
In nature, transport of heat is accomplished in three ways: molecularly (as a result of thermal motion of molecules), by convection (displacement of macroscopic elements of the system in space), and by thermal radiation. The first two are observed only in the presence of material medium (i.e., an ensemble of microparticles such as molecules and atoms in a state of thermal collisions). If geometrically small elements of the medium possess the same properties as their macroscopic aggregate, then the medium is said to be a continuum. Solids and liquids fit well the concept of continuum. Gas can also be considered as a continuous medium if the Knudsen number Kn = 1/L < 10−3 (i.e., if the free path length 1 of molecules is small compared to the characteristic geometrical dimension L of the heat transfer system). For air 1  2.27·10−5T/p, m, where T is the absolute temperature k and p the pressure, Pa. Heat transfer in continua is tackled using a phenomenological approach, making it possible to consider only the resultant characteristics of heat transfer for an entire ensemble of molecules. A distinction needs to be made between single phase and multiphase media. Examples of multiphase media are gas- and vapor-liquid mixtures and dust-laden gases. In certain cases, loose materials and dispersed media (multiphase media the condensed phases of which are in a finely dispersed state) can be regarded as continuous media with assigned effective properties.
2.27·10−5T/p, m, where T is the absolute temperature k and p the pressure, Pa. Heat transfer in continua is tackled using a phenomenological approach, making it possible to consider only the resultant characteristics of heat transfer for an entire ensemble of molecules. A distinction needs to be made between single phase and multiphase media. Examples of multiphase media are gas- and vapor-liquid mixtures and dust-laden gases. In certain cases, loose materials and dispersed media (multiphase media the condensed phases of which are in a finely dispersed state) can be regarded as continuous media with assigned effective properties.
Molecular heat transfer in a continuum is known as heat conduction. The phenomenological description of heat conduction is based on a fundamental hypothesis of heat transfer, Fourier's law,

where λ, W/(mK), is the physical parameter (property) of the medium that is known as the coefficient of thermal conductivity. For many substances such as solids and ordinary liquids λ only depends slightly on temperature and in many cases can be considered a constant. In the case of gases, particularly, in states close to the saturation curve or the critical point, λ strongly depends on temperature and often this cannot be neglected. In isotropic bodies (media) the thermal conductivity does not depend on the heat propagation direction. Many widely encountered solid materials possess anisotropy (i.e., their heat conduction depends on the heat flux direction). Examples are reinforced concrete, fabrics, composite materials. For these media, Fourier's law is applied in a more general statement. It should be noted that heat transfer by heat conduction in most bodies, except carbon and metals, is inefficient and produces a high thermal resistance to heat flux. Therefore, materials with low λ are widely used for thermal insulation of dwellings, premises, and engineering objects. The system in which heat transfer occurs by heat conduction often consists of several bodies close together. Examples are multilayer walls, an iron, or a soldering iron contacting with heated items. In these cases, in addition to heat conduction inside each body of the system, the rate of heat transfer can be also essentially affected by direct contact heat transfer whose characteristics depend on physical processes responsible for heat transport via contacting body surfaces. In fluid media (liquids and gases) under terrestrial conditions heat transfer by heat conduction alone is rarely realized because the temperature field arising due to thermal expansion changes the medium density and gives rise to Archimedean forces (buoyancy forces) which cause free convection.
Convective heat transfer is characteristic of fluid media (fluids), which include liquids, gases, vapors and multiphase fluid media. Depending on which way the fluid flow is implemented, we distinguish between forced convection, which is maintained by supplying to the flow the needed quantity of mechanical energy from the outside, for instance, from the pump or a fan, and free convection as mentioned above. A forced flow under conditions of heat transfer appreciably affected by buoyancy is referred to as mixed convection. Heat transfer caused by the joint action of convective and molecular transfer is said to be convective heat transfer. For practical purposes, the most important case of heat transfer is convective heat exchange between a flowing medium and its interface with another medium, this interface being called a heat transfer surface or a wall in the case where the other medium is a solid. In heat exchangers, overall heat transfer, that is heat exchange between two flowing media across a solid separating wall, plays an essential role. For quantitative expression of convective heat transfer and overall heat transfer, a linear Newton-Richmann relation

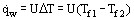
is widely used, where α, W/(m2K), and U are respectively the heat transfer and overall heat transfer coefficients. In contrast to α, the coefficient U takes into account not only the rate of fluid heat transfer by convection, but a thermal resistance of separating wall as well. Relations (2) are applied for both heat transfer surface elements (local heat transfer) and the entire surface on the average (average heat transfer). Average values are commonly used for the whole exchanger (see Mean Temperature Difference). It should be born in mind that the heat transfer coefficient is most frequently not a constant and mayhave an intricate dependence of process parameters, including  and ΔT. For boiling, vapor generation inside a tube, and intensive heating of a turbulent flow gas through a tube (especially at supercritical pressure) sharp reductions in heat transfer coefficient may be observed when qw or ΔT increases. This process is known as heat transfer crisis (burnout). In consideration of internal heat transfer in porous bodies, i.e., a convective heat transfer between a solid matrix and a fluid filtered through it, a quantitative relation
and ΔT. For boiling, vapor generation inside a tube, and intensive heating of a turbulent flow gas through a tube (especially at supercritical pressure) sharp reductions in heat transfer coefficient may be observed when qw or ΔT increases. This process is known as heat transfer crisis (burnout). In consideration of internal heat transfer in porous bodies, i.e., a convective heat transfer between a solid matrix and a fluid filtered through it, a quantitative relation
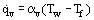
is often used, where  is the heat flux per unit volume of the porous body, Tw the local temperature of the solid skeleton, and Tf the local temperature of fluid. The quantity αv, W/(m3K), is known as the volumetric heat transfer coefficient.
is the heat flux per unit volume of the porous body, Tw the local temperature of the solid skeleton, and Tf the local temperature of fluid. The quantity αv, W/(m3K), is known as the volumetric heat transfer coefficient.
Convective heat transfer between a fluid and a wall is more intensive than for heat conduction alone owing to the additional convective heat transfer which is most intensive in the turbulent fluid flow regime where, due to turbulent mixing, the effective thermal conductivity of fluid sharply increases. Heat transmission deep into the fluid flow can be artificially intensified using various techniques of heat transfer augmentation, e.g., by making the wall rough, implementing secondary flows, and installation of promoters and porous elements (see Augmentation of Heat Transfer).
Convective heat transfer occurs both in external flow over bodies and in internal fluid flow. In the case of external flow over a body with fairly large geometrical dimensions and with high velocity, the resultant characteristics of heat transfer depend only on the processes occurring in a thin fluid layer, directly adjacent to the surface in the flow, that is called a Boundary Layer; owing to this such heat transfer is often called Boundary Layer Heat Transfer. In the case of internal flow which is often encountered in tubes and channels, the dynamic and thermal influence of the wall gradually, with increasing distance from entrance, extends to the entire fluid flow. Internal heat transfer is characterized by a dramatic variation of heat transfer at the entrance region of the channel and its subsequent stabilization far from entrance. Description of the mechanisms of heat transfer at entrance regions of channels is often referred to as the Graetz–Nusselt problem by the names of scientists who were the first to solve this problem for the case of laminar flow (Poiseuille flow) in a tube. Heat transfer in fluid flows having a free surface, such as flows in open beds and falling liquid films, form a special range of problems of great consequence for engineering.
In engineering convective heat transfer often involves physicochemical transformations in the fluid. Of great interest is heat transfer in boiling (pool boiling and forced flow in pipes), evaporation, and film and dropwise condensation that are widely practised in power engineering and underlie many technologies. Convective heat transfer is commonly used in high-temperature aerospace and chemical engineering if chemical reactions proceed in the fluid flow and on the wall. The specific feature of these processes is an additional transmission of latent heat of transformation resulting from molecular and turbulent diffusion of chemical components of the medium. The specific features of hydrodynamics of conducting fluids in a magnetic field (see Magnetohydrodynamics) and liquids in which the laws of viscous friction differ from those of normal (rheological or non-Newtonian liquids) are responsible for the relevant characteristic properties of the mechanism of convective heat transfer.
Heat transfer by radiation occurs by the transmission of electromagnetic waves (photons) in the infrared, visible, and ultraviolet ranges. Such waves are constantly generated by substances as a result of molecular and atomic vibrations due to internal energy. Heat transfer by radiation often becomes dominant in heat transfer in optically transparent systems at temperatures above 1000°C. Radiative heat transfer involves the events of radiation, transport, and absorption of thermal energy. The resultant heat flux released or absorbed by the surface, which for simplicity we consider optically nontransparent, includes the radiation and absorption processes
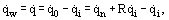
where 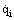 is the density of radiant flux incident from the outside on the surface,
is the density of radiant flux incident from the outside on the surface,  the effective radiation density of the surface itself consisting of both natural radiation
the effective radiation density of the surface itself consisting of both natural radiation 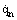 and the reflected part of incident radiation (R is the reflection factor). In contrast to heat conduction and convective heat transfer, no material medium is needed for radiative heat transfer to be accomplished because electromagnetic waves also can easily propagate in vacuum.
and the reflected part of incident radiation (R is the reflection factor). In contrast to heat conduction and convective heat transfer, no material medium is needed for radiative heat transfer to be accomplished because electromagnetic waves also can easily propagate in vacuum.
Combined heat transfer by radiation and heat conduction or by radiation, heat conduction, and convection is implemented in modern engineering objects. Convective and radiative-convective heat transfer is frequently closely interrelated with conductive heat transfer in the wall and, therefore, must be considered jointly. These processes are said to be conjugate.
Heat transfer as an independent field of science has been developing for over three hundred years, but it eventually became an independent science with its own methods, and terms early in the 20th century. The vigorous development of heat transfer science is linked with the development of power engineering, first thermal and in recent years atomic, as well as the development of chemical, aerospace, and cryogenic engineering. Protection of the environment is within the scope of this science. It is no exaggeration to say that in any sphere of industry, a proper implementation of heat transfer based on scientific knowledge of its mechanism ensures a positive effect and saving of fuel, energy, and resources. A powerful impetus to extension of the sphere of application of the heat transfer theory was given by computer engineering. We can state with assurance that an independent domain has been created that is aimed at developing the techniques of numerical heat transfer and the software for it. In accordance with classification of heat transfer processes by the predominating transmission mechanism the science of heat transfer falls into three independent divisions; the theories of heat conduction, convective heat transfer, and radiative heat transfer, respectively.
What the three divisions have in common is the phenomenological approach mentioned above and extensive use of the methods of similarity and dimensional theory for analyzing, quantitative description (the so-called generalized relationships or dimentionless numbers), and simulation of multiple-factor heat transfer processes. The similarity method enables the transformation of the functional dependence between the dimensional physical quantities that are essential for the process considered into a generalized dependence (a similitude relationship) between the dimensionless variables, i.e., similarity numbers or criteria the number of which is less than the number of original dimensional quantities by the number of quantities with independent dimensions (the Buckingham II-theorem) (see Dimensional Analysis). By tradition the most important and generally used similarity numbers are named after the scientists who made a significant contribution to the theory of heat and mass transfer. Such are the Fourier (Fo), Biot (Bi), Reynolds (Re), Nusselt (Nu), Prandtl (Pr), Stanton (St), Peclet (Pe), Grashof (Gr), Rayleigh (Ra), Mach (Ma), Lewis (Le), Schmidt (Sc), Sherwood (Sh), and other numbers. The similarity methods gained currency in the theory of heat conduction and convective heat transfer. Currently efforts are being made to use them in the field of radiative heat transfer.
The theory of turbulent convective heat transfer has made impressive headway owing to the method of statistical averaging of turbulent flow parameters elaborated by O. Reynolds late in the 19th century and applied by him to derive the differential equations of turbulent fluid flow, which were given his name, and the fundumentals of the theory of boundary layer which were formulated by L. Prandtl in 1904. Prandtl also put forward a fruitful model of turbulent transmission, viz., the mixing length model, which enabled a number of classic solutions to turbulent heat transfer problems to be obtained which are fairly consistent with the experimental data. The continued interest shown in improved models of turbulent transfer is accounted for by the fact that the Reynolds averaged equations are not formally closed.
REFERENCES
Gröber, H., Erk, S., and Grigull, U. (1963) Die Grundgesetze der Wärmeübertragung. Springer, 3. Aufl. Berlin.
Kline, S. J. (1965) Similitude and Approximation Theory, McGraw-Hill, New York. DOI: 10.1016/0016-0032(66)90025-1
Kays, W. M. and Crawford, M. E. (1980) Convective Heat and Mass Transfer, McGraw-Hill, New York,
Cebeci, T. and Bradshaw, P. (1984) Physical and Computational Aspects of Convective Heat Transfer, Springer, New York.
Siegel, R. and Howell, J. R. (1972) Thermal Radiation Heat Transfer, McGraw-Hill, New York.
References
- Gröber, H., Erk, S., and Grigull, U. (1963) Die Grundgesetze der Wärmeübertragung. Springer, 3. Aufl. Berlin.
- Kline, S. J. (1965) Similitude and Approximation Theory, McGraw-Hill, New York. DOI: 10.1016/0016-0032(66)90025-1
- Kays, W. M. and Crawford, M. E. (1980) Convective Heat and Mass Transfer, McGraw-Hill, New York,
- Cebeci, T. and Bradshaw, P. (1984) Physical and Computational Aspects of Convective Heat Transfer, Springer, New York.
- Siegel, R. and Howell, J. R. (1972) Thermal Radiation Heat Transfer, McGraw-Hill, New York. DOI: 10.1002/aic.690180243