The fugacity of a component in a mixture is defined as:
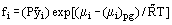
where μi is the chemical potential of component i in the mixture, (μi)pg is the chemical potential of the hypothetical perfect gas at the same pressure, P, temperature, T, and composition (mole fraction) of the mixture,
 .
.
 is the universal gas content.
is the universal gas content.
Fugacity has the dimensions of pressure. In many ways, it behaves in the same way as does partial pressure in a perfect gas mixture, although one must be careful not to take the analogy too far. It is most frequently used when discussing the thermodynamic properties of real gases and rather less so when considering liquids.
If fugacity is used to represent nonideality in both gas and liquid phases, then the condition for equilibrium becomes equality of fugacity for each component in both phases.