Dispersed flow is characterized by the flow where one phase is dispersed in the other continuous phase. This flow configuration is observed in all types (gas-liquid, gas-solid, liquid-liquid and liquid-solid) of two-phase flows as shown in Figure 1.
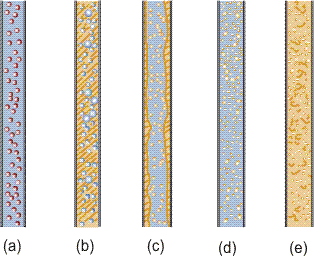
Figure 1. Dispersed flows, (a) gas-solid, liquid-solid, (b) bubbly, (c) annular dispersed, (d) droplet, (e) liquid-liquid.
In Gas-solid and Liquid-Solid Flows, the dispersed phase is always in solid phase because solid particles never coalesce with each other. On the other hand, in Gas-Liquid and Liquid-Liquid Flows, the dispersed phase is determined mainly by the flow rates of both phases since the interface between both phases is deformable, and the dispersed phase coalesces and finally becomes the continuous phase as flow rate increases. For example, in gas-liquid flow the gas phase is dispersed (bubbly flow, Figure 1b) when the gas flow rate is small compared with liquid flow rate. On the other hand the liquid phase is dispersed (annular dispersed, Figure 1c, and droplet flow, Figure 1d) when the liquid flow rate is small compared with gas flow.
However, even in the gas-solid and liquid-solid flows, solid particles agglomerate when the solid flow rate increases as shown in Figure 2. In this case, the group of solid particles behave like in the continuous phase and therefore, such flow configuration is called plug flow (sometimes, actually plugs the pipe) and is distinguished from dispersed flow.
One of the most important features common to all types of dispersed flows is that mass, momentum and energy transfer between the phases are carried out from each particle (here, particle means solid particle, bubble, droplet in gas and liquid) to the surrounding continuous phase. Therefore, the mechanisms of mass, momentum and energy transfer from a single particle basically control the interaction between phases, although, of course, multiparticle effects must be considered. The correlations for phase interactions are usually based on that for a single particle, with some modification, due to the multiparticle effect of volume fraction or mass fraction of the dispersed phase.
Among the interaction terms between phases, the most important one is the drag force acting on the particle, since this reflects two-phase flow effects in determining the flow fields of the dispersed and continuous phases. Drag force, FD, is given in terms of drag coefficient, CD, which is defined by:
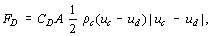
where ρ is the density; u, velocity; suffixes c and d denote the continuous and dispersed phases; and A is the projected area of a particle in its flow direction.
For gas-solid and liquid-solid flows, the correlation of drag coefficient for a single spherical particle is used although in actual systems, the solid particle is not necessarily spherical. Schiller & Naumann (1933) have expressed it as:
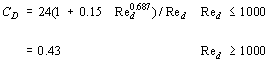
where Red is the Reynolds Number based on the particle diameter. In dispersed flow, the effect of the multiparticle system is given by Bouilard (1989) as:

where
 is the drag coefficient of multiparticle system and εd is the volume fraction of the dispersed phase.
is the drag coefficient of multiparticle system and εd is the volume fraction of the dispersed phase.
For gas-liquid and liquid-liquid flows, the effect of the deformation of particle (bubble or droplet) on the drag coefficient must be considered, particularly for larger particles. For smaller particles, the effects of the deformation are small and Eq. (1) is approximately applied, although some modification is needed to consider the viscosities of both phases (Hadamard, 1911). However, as the particle diameter increases, the deformation becomes appreciable and the effects of deformation on drag coefficient become predominant. The drag coefficient is given by Harmathy (1960) as:

where Δρ is the difference in density between the phases, Dp the particle diameter and σ the interfacial surface tension. This drag coefficient gives the constant terminal velocity for deformable particle (bubble or droplet), which is given by:
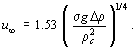
The drag coefficient is taken from the maximum of the values given by Eq. (2) and (4). The effects of the multi-particle system on gas-liquid and liquid-liquid systems have been studied by Ishii and Chawla (1979). For smaller particles the drag coefficient of which is given by Eq. (2), the drag coefficient of the multiparticle system is given by:

n = 1 for bubble in liquid; n = 1.75 for drop in liquid; n = 2.5 for drop in gas.
For larger particles, the drag coefficient of which is given by Eq. (4),


m = 1.5 for bubble in liquid; m = 2.25 for drop in liquid; m = 3 for drop in gas.
In applying the drag coefficients for dispersed flow discussed above, there are two approaches, i.e., Eulerian and Lagrangian.
In the Eulerian approach, both continuous and dispersed phases are averaged in appropriate time and/or space domains. The resulting basic equations of mass, momentum and energy conservation are expressed in Eulerian coordinate. As a result of averaging, the dispersed phase is also treated as a kind of continuous fluid. Depending upon the model which regards two-phase flow as a single mixture fluid or two separate fluids, there are two types of formulations called mixture model and two-fluid model (Ishii, 1975; Delhaye, 1968). Such an Eulerlian approach and basic equations in dispersed flow are the same as those in other types of two-phase flows.
For example, the momentum conservation equation based on two-fluid model is:

where suffix k denote dispersed (k = d) and continuous (k = c) phases and the upper bar denotes averaged value. In Eq. (9), Mk represents the momentum transfer term between phases. Using the drag coefficient in a multiparticle system (
 derived above), this is given by:
derived above), this is given by:


Using this momentum equation with mass and energy equations in the Eulerian approach, the averaged behavior of heat, mass and momentum transfer in dispersed flow can be analyzed.
In the Lagrangian approach, the motion of each particle is calculated in Lagrangian coordinate, whereas the continuous phase is treated in Eulerian coordinate. Using the drag coefficient of particles in a multiparticle system, the trajectory of each particle motion is calculated using the equation of motion of particle:

where mp is the mass of single particle and fd is the force acting upon the particle, such as gravity, pressure force and collision between particles, etc. Here, it should be noted that velocities uc and ud are local instantaneous (not averaged) velocities of particle and continuous phase. When the flow of continuous phase is turbulent, uc is not given by the solution of averaged momentum equation [Eq. (9)]. In order to get uc, appropriate Eulerian and Turbulence Models, such as direct numerical simulation (DNS), large-eddy simulation (LES), two-equation model (k−ε, k−kL models), etc. are needed. This Lagrangian approach reflects physical mechanisms of dispersed flow more precisely. However, there are numerous dispersed particles in actual dispersed flow so that simulations of all particles are almost impossible with the present capacity of large-scale computers. Therefore, only simulation of sample particles is carried out and, using some statistical assumptions, the flow behavior and heat and mass transfer of dispersed flow are evaluated. Such Lagrangian approach is carried out particularly in the area of gas-solid dispersed flow (Yuu et al., 1978).
Along with Lagrangian analysis of particle behavior in dispersed flow, many experimental studies have also been carried out for various parameters of dispersed flow. Among these, Eulerian and deposition rate of particles on the pipe wall is quite important and common to all types of dispersed flow. The deposition rate,
 , is defined as the mass flux of dispersed particle deposited on the wall per unit of time and unit area. This quantity is related to the mass concentration of dispersed phase in the continuous phase, C. The coefficient relating deposition rate and concentration is called deposition coefficient (kD) and is defined by:
, is defined as the mass flux of dispersed particle deposited on the wall per unit of time and unit area. This quantity is related to the mass concentration of dispersed phase in the continuous phase, C. The coefficient relating deposition rate and concentration is called deposition coefficient (kD) and is defined by:

There are many correlations of deposition coefficient for gas-solid and gas-liquid (droplet or annular droplet) flows.
In real gas-solid flow, a particle diameter is relatively small. Therefore, the particle follows Brownian motion (particle diameter less than 1 micron) and turbulent motion of continuous phase (less than 10 microns). Experiments and analyses have been carried out and correlations have been derived (Beal S.K., 1970). A typical result of deposition coefficient of gas-solid flow is shown in Figure 3.
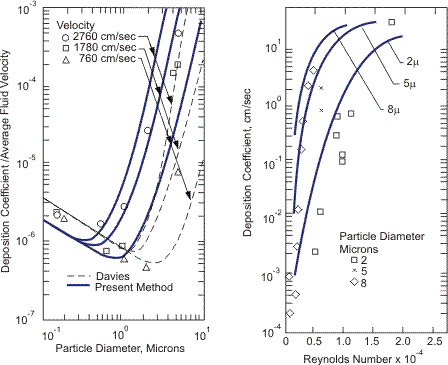
Figure 3. Deposition coefficient of solid in gas (Beal S.K., 1970). Nuclear Science and Engineering, 40, p. 8, with permission.
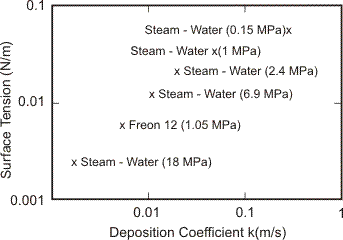
In gas-liquid flow, particle (droplet) diameter is relatively large (10 to 100 microns) and droplet may deform due to hydrodynamic force. In this case, the mechanisms of deposition are quite complicated. Therefore, the derived correlations are based mainly on experimental data of droplet deposition. The earliest and standard correlation for deposition coefficient has been derived at Harwell Laboratory, UK (Whalley et al., 1974), shown in Figure 4. More recently, the correlation considering turbulence droplet interaction has been presented by McCoy & Hanratty (1977).
REFERENCES
Bouillard, J. X. et al. (1989), Porosity distributions in a fluidized bed with an immersed obstacleAIChE J., 35, 908.
Delhaye, J. M. (1968) Equations Fondamentales des Ecoulements Diphasiques, CEA-R-3429.
Beal, S. K. (1970) Nuclear Sci. & Eng., 40, I.
Ishii, M. (1975) Themo-fluid Dynamic Theory of Two-Phase Flow, Eyrolles Paris.
Ishii, M. and Chawla, T. C. (1979), Local drag laws in dispersed two-phase flow, Argonne. National Laboratory Report, ANL 79-105.
Hadamard, J. (1911) Compt. Rend. Acad. Sci. Paris, 52, 1735.
Harmathy, T. Z. (1960), Velocity of large drops and bubbles in media of infinite or restricted extent. AIChE J., 6, 281.
McCoy, D. D. and Hanratty, T. J. (1977), Rate of deposition of droplets in annular two-phase flow, Int. J. Multiphase Flow, 3, 319. DOI: 10.1016/0301-9322(77)90012-X
Schiller, L. and Naumann, A. (1933) Z. V.D.I., 77, 318.
Whalley, P. B. et al. (1974) Proc. 5th Int. Heat Transfer Conf., Versailles, Vol. 4, p. 290.
Yuu, S. et al. (1978), Particle turbulent diffusion in a dust laden round jet, AIChE J., 24, 509.
References
- Bouillard, J. X. et al. (1989), Porosity distributions in a fluidized bed with an immersed obstacleAIChE J., 35, 908. DOI: 10.1002/aic.690350604
- Delhaye, J. M. (1968) Equations Fondamentales des Ecoulements Diphasiques, CEA-R-3429.
- Beal, S. K. (1970) Nuclear Sci. & Eng., 40, I.
- Ishii, M. (1975) Themo-fluid Dynamic Theory of Two-Phase Flow, Eyrolles Paris.
- Ishii, M. and Chawla, T. C. (1979), Local drag laws in dispersed two-phase flow, Argonne. National Laboratory Report, ANL 79-105.
- Hadamard, J. (1911) Compt. Rend. Acad. Sci. Paris, 52, 1735.
- Harmathy, T. Z. (1960), Velocity of large drops and bubbles in media of infinite or restricted extent. AIChE J., 6, 281. DOI: 10.1002/aic.690060222
- McCoy, D. D. and Hanratty, T. J. (1977), Rate of deposition of droplets in annular two-phase flow, Int. J. Multiphase Flow, 3, 319. DOI: 10.1016/0301-9322(77)90012-X
- Schiller, L. and Naumann, A. (1933) Z. V.D.I., 77, 318.
- Whalley, P. B. et al. (1974) Proc. 5th Int. Heat Transfer Conf., Versailles, Vol. 4, p. 290.
- Yuu, S. et al. (1978), Particle turbulent diffusion in a dust laden round jet, AIChE J., 24, 509. DOI: 10.1002/aic.690240316