RADIATIVE COOLING AND SOLIDIFICATION OF CORE MELT DROPLETS
Following from: Near-infrared properties of droplets of an aluminum oxide melt; Thermal radiation from nonisothermal spherical particles; Thermal radiation from nonisothermal particles in combined heat transfer problems
Leading to: Thermal radiation modeling in melt-coolant interaction
The problem of melt-water interaction is especially important for analysis of hypothetical severe accidents in light water nuclear reactors. A severe accident involves melting of the core and possible subsequent interaction of the core melt (UO2-ZrO2 composition) with water. The fuel-coolant interaction (FCI) may lead to steam explosion with a significant part of the melt thermal energy converted into the mechanical energy of the detonation wave (Corradini et al., 1988; Fletcher and Anderson, 1990; Fletcher, 1995; Theofanous, 1995; Berthoud, 2000). Physical processes that govern steam explosion energetics are multifaceted and complex (Fletcher, 1995; Dinh et al., 1999a; Dinh, 2007). The probability of steam explosion is determined by the so-called premixing stage of the FCI when the melt jets are fragmented into numerous small droplets. The fragmentation of the melt jets and large droplets is considered as very important process (Bürger, 2006). Many experiments with core melt jets have failed to produce strong steam explosions. On the contrary, spontaneous explosions have been observed when Al2O3 melt jets are employed. It appears that the explosivity of the core melt is extremely low (Huhtiniemi et al., 1999). One can assume that this result is explained by different conditions of droplet fine fragmentation because of the different character of cooling and solidification of core melt and alumina particles. Simple estimates show that thermal radiation is the main mode of heat transfer from a single particle to ambient water (Dinh et al., 1999b; Fletcher, 1999; Dombrovsky, 1999). For this reason, it is interesting to focus on the radiative cooling of these particles.
The main part of the radiation emitted by particles is absorbed in ambient water, at least in the case of a not-too-high volume fraction of particles (Dombrovsky, 2003). This allows us to assume that radiation heat transfer between the particles is insignificant compared to local heat transfer to surrounding water, and consider a model problem for single particles.
There is no spherical symmetry of the heat transfer problem, even in the case of the almost spherical shape of the melt droplet, because of the considerable variation of the flow parameters along the surface of the moving particle. Nevertheless, simple estimates showed that the total heat flux from the hot particle is almost symmetric because the contribution of convection is relatively small. The increasing role of convection for small particles cannot also result in considerable asymmetry of the particle temperature field because of the insignificant temperature difference in small particles.
The 1D mathematical problem statement for a single semitransparent spherical particle is based on the following transient energy equation with the usual boundary and initial conditions:

|
(1a) |

|
(1b) |
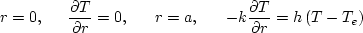
|
(1c) |
where h is the average convective heat transfer coefficient for the heat transfer from a hot particle to ambient water through a thin steam layer, P(t,r) is the heat loss rate due to thermal radiation, and T0 is the uniform temperature of the melt droplet in the beginning of cooling. At this stage of the modeling, we ignore the difference between the temperatures of melting and solidification, assuming the phase change to be localized at the isotherm T = Tm. This means that we consider only pure substances or their eutectics. In the general case, one should take into account the difference between the solidus and liquidus temperatures of the mixtures. We do not consider the effect of the melt undercooling and subsequent spontaneous solidification. As was discussed recently by Tseng and Viskanta (2005), this effect may take place in the case of internal cooling of semitransparent melt droplets. We also neglect the change of density ρ during the solidification of the melt.
The usual melt particles are large in comparison with the infrared radiation wavelength. This is true even for small particles arising behind the shock wave (Fletcher, 1988; Abe et al., 2006; Magallon, 2006). Therefore, one can ignore electromagnetic wave effects in the analysis of the radiation field inside the particle. We assume also that the thickness of the steam layer at the particle surface is much greater than the radiation wavelength. In this case, the radiation intensity calculations can be based on the traditional radiation transfer theory, not only inside but also outside the particle (Dombrovsky, 2000).
Strictly speaking, the problem should be considered as a spectral one. In the present analysis, we use the so-called gray model assuming the index of refraction n and absorption coefficient α(T) of the particle substance near the melting temperature to be independent of the wavelength. The latter assumption will be justified below. The heat transfer problem under consideration is a conjugated problem because heat transfer conditions on a particle surface depend on the temperature field in the particle. Following a recent paper by Dombrovsky and Dinh (2008), we use the fixed constant value of convective heat transfer coefficient h = 300 W/(m2K).
It is interesting to compare the numerical results obtained by use of the radiative-conductive model described above with predictions based on a relatively simple isothermal model. The energy equation for an isothermal particle can be written in the following form:

|
(2) |
The integral emissivity ε is practically the same as that of a semitransparent particle in vacuum, and can be approximated as follows (Dombrovsky, 2002):

|
(3) |
The isothermal approximation applicability is analyzed below.
Two metal oxides are considered in this article, namely, aluminum oxide Al2O3 and corium, which consists mainly of uranium dioxide UO2 and small amount of zirconium dioxide ZrO2. Aluminum oxide is chosen as a representative of light-weight oxides that are used in the model experiments on steam explosion.
The index of refraction of aluminum oxide is insensitive to the temperature, and the spectral variation of this index in the most important near-infrared range is insignificant (Dombrovsky, 1982, 1996). In the same spectral range, the index of absorption κ at temperatures near the melting point increases approximately linearly with the wavelength (Mularz and Yuen, 1972; Lingart et al., 1982; Rubtsov et al., 1984). These facts can be considered as a basis of the gray model (the values n and α = 4κ/λ do not depend on λ) and the assumption about a constant (independent of temperature) index of refraction. The experimental data for the absorption coefficient of aluminum oxide near the melting temperature published in the known papers are very different, but in all cases a significant increase in absorption with temperature is observed (see also the article Near-infrared properties of droplets of aluminum oxide melt). According to Dombrovsky (2007a) and Dombrovsky and Dinh (2008), the following approximation of the temperature dependence of the absorption coefficient is used:
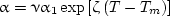
|
(4) |
where α1 = 837 m-1, ζ = 0.005 K-1. Values ν = 1 and ν = 3 (called the variants 1 and 2 in the figures below) correspond to the experimental data by Rubtsov et al. (1984) and Mularz and Yuen (1972). The values of other physical parameters of aluminum oxide near the melting temperature are given in Table 1.
Table 1. Physical parameters of aluminum oxide
| Tm, K | 2320 |
| ρ, kg/m3 | 3000 |
| c, J/(kg K) | 1400 |
| k, W/(m K) | 2 |
| L, kJ/kg | 1070 |
| n | 1.82 |
It is convenient to present the results of the calculations by using the dimensionless variables. Particularly, introducing the dimensionless temperature θ = (T -Te)/(Tm -Te) and taking into account that Te << Tm, we can ignore the temperature of water Te. The other dimensionless parameters are as follows:

|
(5) |
The results of calculations for alumina particles are presented in Figs. 1 and 2. The initial overheating of the melt was assumed to be θ0 = 1.02. One can see a significant difference between the results for two variants of the absorption coefficient. For the first variant, the optical thickness of the particle is small, and thermal radiation leads to almost simultaneous solidification of the melt in a thin surface layer and in the center of the particle. For the second variant, the optical thickness is much greater, and the particle is solidified from the surface. The calculations showed that the surface solidification rate is not very sensitive to the great uncertainty in the absorption index of aluminum oxide. The surface solidification rate is controlled by the convective heat transfer coefficient, and varies from 0.2 to 0.35 mm/s with the convective heat transfer coefficient from 200 to 400 W/(m2 K).
 |
 |
Figure 1. Temperature profiles in aluminum oxide particle of radius a = 1 mm: (a) variant 1; (b) variant 2.
 |
Figure 2. Profile of radiative heat losses in an aluminum oxide particle of radius a = 1 mm at time moment t = 0.2 s.
It is interesting to compare the numerical results obtained on the basis of the radiative-conductive model with calculations in the isothermal approximation. One can see in Figs. 3 and 4 that the isothermal model gives a rather good estimate of the average particle temperature and the total radiation flux to the ambient water, excluding the period of solidification. The integral parameters of the solidification process including the time of complete solidification ts can be estimated on the basis of the isothermal model, but one should solve the radiative-conductive problem to find more accurate values. The latter statement is illustrated in Table. 2. One can see that the isothermal model overestimates ts (37%) for the first variant, and underestimates ts (20%) for the second variant of the absorption coefficient.
 |
 |
Figure 3. Time variation of temperature of an aluminum oxide particle (a = 1 mm): (a) variant 1; (b) variant 2; 1, in the center of the particle; 2, on the particle surface; 3, isothermal model.
 |
Figure 4. Integral radiation flux from aluminum oxide particle of radius a = 1 mm: (a) variant 1; (b) variant 2; 1, radiative-conductive model; 2, isothermal model.
Table 2. Solidi cation time for a particle of radius a = 1 mm
| ts, s | ||
| Variant 1 | Variant 2 | |
| Radiative-conductive model | 0.46 | 0.69 |
| Isothermal model | 0.63 | 0.55 |
To the best of our knowledge, there is no data for near-infrared optical constants of corium and its main component, uranium dioxide. The data for the optical properties of zirconium dioxide, which is also a component of corium, are very limited (Wood and Nassau, 1982; Cabannes and Billard, 1987; Petrov and Chernyshev, 1999; Akopov et al., 2001; Dombrovsky et al., 2007). There is no data for binary mixtures of uranium dioxide and zirconium dioxide. Experimental data by Bober et al. (1981, 1984) for UO2 were obtained in the visible range, 0.45 ≤ λ ≤ 0.75 μm, where this substance is opaque. The value κ = 0.8 from (Bober et al., 1981, 1984) corresponds to an absorption coefficient α ~ 107 m-1 which is four orders of magnitude greater than the absorption coefficient of aluminum oxide. It is known that absorption coefficient of uranium dioxide strongly decreases by transfer from the visible to the near-infrared spectral range, but this coefficient remains much greater than that for aluminum oxide. In a paper by Anderson (1976), the Rosseland average value α = 5 × 103 m-1 is given for a UO2 melt. By using the last value of the absorption coefficient, we find the optical thickness of a particle of radius 1 mm equal to τ = 5. It is sufficient to consider this particle of corium as an opaque particle in the near-infrared range. The recent study of the spectral absorption of UO2 in the temperature range from room temperature up to 1173 K reported by Ruello et al. (2004) confirms the high absorption in the visible and near-infrared spectral range. It was also shown that the boundary of the strong optical absorption band moves to the infrared range when the sample is heated. The model problem for an opaque particle is reduced to the following one:

|
(6a) |

|
(6b) |

|
(6c) |
The integral hemispherical emissivity of bulk corium is assumed to be equal to εb = 0.85 (Harding et al., 1989; Fink, 2000). The same value is used instead of Eq. (3) in the isothermal approximation. The values of other physical parameters of corium near the melting temperature are given in Table 3.
Table 3. Thermophysical properties of corium
| Tm, K | 2850 |
| ρ, kg/m3 | 8000 |
| c, J/(kg K) | 600 |
| k, W/(m K) | 3 |
| L, kJ/kg | 400 |
The calculated temperature profiles in a corium particle are shown in Fig. 5. The surface layer of the particle is cooled much faster than that in the case of an aluminum oxide particle. To understand the cause of this effect, the additional calculation for an aluminum oxide particle in the opaque limit (by ignoring the semitransparency of this particle) is also presented in Fig. 5.
 |
Figure 5. Temperature profiles in opaque particles of radius a = 1 mm: I, opaque limit for alumina particle; II, corium particle.
It is clear that the fast surface solidification of a corium particle is only partially explained by higher temperature. The effect of particle opacity is also significant (compare with profiles in Fig. 1). The front of solidification moves with approximately constant velocity to the particle center, and the total solidification time appears to be relatively large for opaque particles (see also Table 4). As one can expect, the isothermal approximation is inapplicable to corium particles due to the great temperature difference between the surface and the center of the particle (see Figs. 6 and 7). Radiative cooling leads to much faster solidification of the melt droplets than is predicted by ignoring the radiation (see Table 4). It is important that the physical picture of solidification of opaque droplets of corium and semitransparent droplets of aluminum oxide is different. The surface crust is formed very fast on the surface of corium droplets because of intense radiative heat loss from the surface. This crust can inhibit subsequent deformation and fragmentation of the droplet. On the contrary, thermal radiation of alumina droplets comes not from the surface, but from the droplet volume. As a result, the radiation practically does not accelerate the formation of the surface crust layer.
Table 4. Solidi cation time for a particle of radius a= 1 mm
| Particle substance | ts, s | |||
| Without radiation | With radiation | |||
| Alumina | 2.82 | Variant 1 | Variant 2 | Opaque limit |
| 0.46 | 0.63 | 1.28 | ||
| Corium | 2.21 | 0.86 | ||
 |
Figure 6. Time variation of the temperature of an opaque corium particle (a = 1 mm): 1, in the center of the particle; 2, on the particle surface; 3, isothermal model.
 |
Figure 7. Integral radiation flux from opaque corium particle of radius a = 1 mm: 1, radiative-conductive model; 2, isothermal model.
Let us return to the more detailed analysis of opaque particle solidification. The transient heat conduction problem (6) can be approximately presented in the following dimensionless form:

|
(7a) |

|
(7b) |

|
(7c) |
It is assumed here that material density, heat capacity, and thermal conductivity are independent of temperature, that the heat transfer coefficient is constant, and that the melting temperature is much greater than the temperature of the water. The problem (7) includes three dimensionless parameters: L = L/cTm, the relative lateral heat of melting; Bi = ha/k, the Biot number; and S = σTm3/h, the parameter characterizing the relative role of thermal radiation in heat losses from the particle. The current Fourier number t = kt/(ρca2) is used in Eq. (7a) as a time variable.
One can see in Fig. 5 that temperature profiles in the solid layer on the particle surface are almost linear at the initial stage of solidification. Therefore, it is essential to consider the following approximation of the temperature profiles in this period:

|
(8) |
where θs(t) = θ(1,t) is the dimensionless surface temperature of the particle, and ri = ri/a is the dimensionless current radius of the solidification front inside the particle. The boundary condition at r = 0 is satisfied. Substitution of Eq. (8) into the boundary condition at r = 1 gives

|
(9) |
Equation (8) should satisfy the equation of thermal balance, which is obtained from integration of Eq. (7a) along the radius,

|
(10) |
where

|
(11) |
After simple transformations, we obtain the following Cauchy problem for coupled ordinary differential equations:

|
(12a) |

|
(12b) |

|
(12c) |
The problem (12) should be solved from t = 0 to t = t s, which is defined by equation ri(ts) = 0. In Fig. 8, the calculated time dependences r i(t), θs(t) for a corium particle are compared with the numerical solution of the conduction problem (7). One can see that the approximate solution gives fairly good results when ri > 0.5, but the error increases in the final period of solidification, and the solidification time is considerably underestimated in this approach. The latter is not so important of a limitation because the initial rate of surface solidification is the main parameter, which influences the possibility of particle fragmentation.
 |
 |
Figure 8. Time variation of solidification front position (a) and surface temperature (b) of a corium particle of radius a = 1 mm: I, without radiation, II, with radiation (solid lines, solution of conduction problem; dashed lines, approximate calculation).
It is instructive to note that in their recent papers, Moriyama et al. (2006) and Pohlner et al. (2006) employed the parabolic model to obtain approximate relations for the temperature difference between the center and the surface of a corium particle. The so-derived approximate relations were recommended for implementation into FCI simulation codes. It is well known that the parabolic model gives good results for quasi-steady heating or cooling of the particle with heat absorption, or generation inside the particle without phase changes (Dombrovsky and Sazhin, 2003a,b; Dombrovsky and Lipiński, 2007). However, the parabolic model is not applicable to a solidifying melt droplet. Instead, one can use the complete conduction problem solution or the above-suggested approximate model to determine the temperature difference in the solidifying corium particles.
One can see in Fig. 8a that the solidification rate  (t) = -
(t) = - i(t) is approximately constant in the range of 0.5 < ri < 1. This enables us to evaluate the solid layer thickness as follows:
i(t) is approximately constant in the range of 0.5 < ri < 1. This enables us to evaluate the solid layer thickness as follows:

|
(13) |
It can be seen that the crust thickness Δ is independent of the particle size, but strongly dependent on the melting temperature. Note that Eq. (13) has been used recently by Dombrovsky (2007b) to analyze the conditions of possible fragmentation of an opaque solidifying particle under the action of pressure drop in an unstable steam layer at the particle surface. A comparison between the pressure drop rate in the steam layer (Dombrovsky and Zaichik, 2000; Alipchenkov et al., 2002) and the surface solidification rate was used to estimate the conditions of the solid crust stability. It was shown that fast surface solidification of corium melt droplets can prevent fine fragmentation of the droplets and decrease the probability of a subsequent steam explosion.
An approximate model for the temperature profile in particles based on a combination of the above approach with the parabolic model for completely solidified particles has been recently suggested by Dombrovsky et al. (2009). This model was implemented in the regular code VAPEX-P for multiphase flow calculations. Consider the main relations of this model. The radial temperature profile in an opaque corium particle during its solidification is approximated as

|
(14) |
with the following Cauchy problem for the surface temperature of the particle and the position of the solidification front:

|
(15a) |

|
(15b) |

|
(15c) |
The only difference of Eq. (15) from the dimensionless formulation (12) is that we do not use now the simplified expression for radiation flux qrad. The value of qrad is determined from a radiative transfer solution in a volume of a semitransparent multiphase medium. The latter problem is considered in the article Thermal radiation modeling in melt-coolant interaction. In the derivation of Eq. (15b), it was assumed that the total heat transfer coefficient ht is almost constant during the particle solidification. Of course, we do not consider the temperature dependence of the heat capacity and thermal conductivity of corium in this model. The problem (15) should be solved from t = 0 to t = tsol, which is defined by the equation, rf(tsol) = 0.
The above approximate model is completed by the parabolic model for the period just after total solidification of the particle,

|
(16) |
At the initial time moment, one can assume Ti to be equal to Ti(tsol) taken from the above-presented solution. Note that there is no rigorous matching condition at t = tsol because it is impossible to satisfy all the physical conditions in this approximation: the conservation of energy and the absence of jumps in heat flux and surface temperature. Fortunately, this is not so important because the temperature difference in a solid particle is much less than that during the particle solidification. After obvious rewriting, one can obtain the following ordinary differential equation for the particle surface temperature:

|
(17) |
After solving the initial-value problem for the surface temperature, one can determine the average (bulk) temperature of the particle,

|
(18) |
The numerical results for realistic FCI reported by Dombrovsky et al. (2009) indicated that the nonisothermicity of corium particles is an important factor, especially for the prediction of the average temperature of the particles. Fortunately, the increase in computational time in comparison with the isothermal model is insignificant (<5%), which is acceptable for practical calculations.
A time variation of the temperature of single corium particles during their cooling and solidification is illustrated in Fig. 9, where the model calculations at a representative constant value of heat transfer coefficient h = 300 W/(m2K) in the limit of the opaque host medium are presented. The surface temperature Ts and average temperature T of the particles were calculated by the above-suggested approximate differential model. Small jumps on the average temperature curves at the moment of solidification completion are not physical. As was discussed above, this effect is explained by the approximate matching of solutions for solidifying and solid particles. One can see in Fig. 9 that the difference between the surface and average temperatures of corium particles during their solidification is significant. This may reach 300 K for particles of diameter d = 2a = 5 mm. Obviously, the uncertainty of the thermal conductivity of corium near the melting temperature is the main source of possible considerable errors. This uncertainty makes reasonable the use of the approximate model for the temperature profile in corium particles.
 |
Figure 9. Comparison between surface temperature (I) and average temperature (II) of corium particles: 1, d = 3 mm; 2, d = 5 mm; 3, d = 7 mm.
REFERENCES
Abe, Y., Matsuo, E., Arai, T., Nariai, H., Chitose, K., Koyama, K., and Itoh, K., Fragmentation Behavior during Molten Material and Coolant Interactions, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 1668-1681, 2006.
Akopov, F. A., Val’ano, G. E., Vorob’ev, A. Yu., Mineev, V. N., Petrov, V. A., Chernyshev, A. P., and Chernyshev, G. P., Thermal Radiative Properties of Ceramic of Cubic ZrO2 Stabilized with Y2O3 at High Temperatures, High Temp., vol. 39, no. 2, pp. 244-254, 2001.
Alipchenkov, V. M., Dombrovsky, L. A., and Zaichik, L. I., The Growth and Stability of Vapor Film on the Surface of a Hot Spherical Particle, High Temp., vol. 40, no. 1, pp. 100-104, 2002 .
Anderson, E. E., Radiative Heat Transfer in Molten UO2 Based on the Rosseland Diffusion Method, Nucl. Tech., vol. 30, pp. 65-70, 1976.
Berthoud, G., Vapor Explosions, Ann. Rev. Fluid Mech., vol. 32, pp. 573-611, 2000.
Bober, M., Singer, J., and Wagner, K., Determination of the Optical Constants of Liquid UO2 from Reflectivity Measurements, Proc. of 8th Symp. Thermophys. Prop., Gaithersburg, MD, vol. II, ASME, pp. 234-244, 1981.
Bober, M., Singer, J., and Wagner, K., Bestimmung der Optischen Konstanten von Geschmolzenen Kernbrennstoffen, J. Nucl. Mater., vol. 124, pp. 120-128, 1984.
Bürger, M., Particulate Debris Formation by Breakup of Melt Jets: Main Objectives and Solution Perspectives, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 1991-1997, 2006.
Cabannes, F. and Billard, D., Measurement of Infrared Absorption of Some Oxides in Connection with the Radiative Transfer in Porous and Fibrous Materials, Int. J. Thermophys., vol. 8, no. 1, pp. 97-118, 1987.
Corradini, M. L., Kim, B. J., and Oh, M. D., Vapor Explosion in Light Water Reactors: A Review of Theory and Modeling, Prog. Nucl. Energy, vol. 22, no. 1, pp. 1-117, 1988.
Dinh, T. N., Material Property Effect in Steam Explosion Energetics: Revisited, Proc. of 12th Int. Topical Meeting Nucl. Reactor Therm. Hydraulics (NURETH-12), Pittsburg, PA, Sept. 30-Oct. 4, Amer. Nucl. Soc., Paper No. 150, 2007.
Dinh, T. N., Bui, V. A., Nourgaliev, R. R., Green, J. A., and Sehgal, B. R., Experimental and Analytical Studies of Melt Jet-Coolant Interactions: A Synthesis, Nucl. Eng. Des., vol. 189, no. 1-3, pp. 299-327, 1999a.
Dinh, T. N., Dinh, A. T., Nourgaliev, R. R., and Sehgal, B. R., Investigation of Film Boiling Thermal Hydraulics Under FCI Conditions: Results of Analyses and Numerical Study, Nucl. Eng. Des., vol. 189, no. 1-3, 251-272, 1999b.
Dombrovsky, L. A., Possibility of Determining the Disperse Composition of a Two-Phase Flow from the Small-Angle Light Scattering, High Temp., vol. 20, no. 3, pp. 472-479, 1982.
Dombrovsky, L. A., Radiation Heat Transfer in Disperse Systems, Begell House, New York and Redding, CT, 1996.
Dombrovsky, L. A., Radiation Heat Transfer from a Spherical Particle via Vapor Shell to the Surrounding Liquid, High Temp., vol. 37, no. 6, pp. 912-919, 1999.
Dombrovsky, L. A., Radiation Heat Transfer from a Hot Particle to Ambient Water through the Vapor Layer, Int. J. Heat Mass Transfer, vol. 43, no. 13, pp. 2405-2414, 2000.
Dombrovsky, L. A., A Spectral Model of Absorption and Scattering of Thermal Radiation by Droplets of Diesel Fuel, High Temp., vol. 40, no. 2, pp. 242-248, 2002.
Dombrovsky, L. A., Radiation Transfer through a Vapour Gap under Conditions of Film Boiling of Liquid, High Temp., vol. 41, no. 6, pp. 819-824, 2003.
Dombrovsky, L. A., Thermal Radiation of Nonisothermal Particles in Combined Heat Transfer Problems (dedication lecture), Proc. of 5th Int. Symp. Radiat. Transfer, Bodrum, Turkey, June 17-22, Begell House, 2007a.
Dombrovsky, L. A., An Estimate of Stability of Large Solidifying Droplets in Fuel-Coolant Interaction, Int. J. Heat Mass Transfer, vol. 50, no. 19-20, pp. 3832-3836, 2007b.
Dombrovsky, L. A., Davydov, M. V., and Kudinov, P., Thermal Radiation Modeling in Numerical Simulation of Melt-Coolant Interaction, Comput. Thermal Sci., vol. 1, no. 1, pp. 1-35, 2009.
Dombrovsky, L. A. and Dinh, T. N., The Effect of Thermal Radiation on the Solidification Dynamics of Metal Oxide Melt Droplets, Nucl. Eng. Des., vol. 238, no. 6, pp. 1421-1429, 2008.
Dombrovsky, L. A. and Lipiński, W., Transient Temperature and Thermal Stress Profiles in Semi-Transparent Particles Under High-Flux Irradiation, Int. J. Heat Mass Transfer, vol. 50, no. 11-12, pp. 2117-2123, 2007.
Dombrovsky, L. A. and Sazhin, S. S., A Parabolic Temperature Profile Model for Heating of Droplets, ASME J. Heat Transfer, vol. 125, no. 3, pp. 535-537, 2003a.
Dombrovsky, L. A. and Sazhin, S. S., A Simplified Nonisothermal Model for Droplet Heating and Evaporation, Int. Comm. Heat Mass Transfer, vol. 30, no. 6, pp. 787-796, 2003b.
Dombrovsky, L. A., Tagne, H. K., Baillis, D., and Gremillard, L., Near-Infrared Radiative Properties of Porous Zirconia Ceramics, Infrared Phys. Tech., vol. 51, no. 1, pp. 44-53, 2007.
Dombrovsky, L. A. and Zaichik, L. I., The Dynamics of Vapor Void Under Conditions of Thermal Interaction of a Hot Spherical Particle with Ambient Water, High Temp., vol. 38, no. 6, pp. 938-947, 2000.
Fink, J. K., Thermophysical Properties of Uranium Dioxide (Review), J. Nucl. Mater., vol. 279, no. 1, pp. 1-18, 2000.
Fletcher, D. F., The Particle Size Distribution of Solidified Melt Debris from Molten Fuel-Coolant Interaction Experiments, Nucl. Eng. Des., vol. 105, no. 3, pp. 313-319, 1988.
Fletcher, D. F., Steam Explosion Triggering: A Review of Theoretical and Experimental Investigations, Nucl. Eng. Des., vol. 155, no. 1-2, pp. 27-36, 1995.
Fletcher, D. F., Radiation Absorption During Premixing, Nucl. Eng. Des., vol. 189, no. 1-3, pp. 435-440, 1999.
Fletcher, D. F. and Anderson, R. P., A Review of Pressure-Induced Propagation Models of the Vapor Explosion Process, Prog. Nucl. Energy, vol. 23, no. 2, pp. 137-179, 1990.
Harding, J. H., Martin, D. G., and Potter, P. E., Thermophysical and Thermochemical Properties of Fast Reactor Materials, Commiss. Eur. Commun. Report EUR 12402 EN, 1989.
Huhtiniemi, I., Magallon, D., and Hohmann, H., Results of Recent KROTOS FCI Tests: Alumina Versus Corium Melts, Nucl. Eng. Des., vol. 189, no. 1-3, pp. 379-389, 1999.
Lingart, Yu. K., Petrov, V. A., and Tikhonova, N. A., Optical Properties of Synthetic Sapphire at High Temperatures. II. Properties of Monocrystal in Opacity Region and Melt Properties, High Temp., vol. 20, no. 6, pp. 1085-1092, 1982.
Magallon, D., Characteristics of Corium Debris Bed Generated in Large-Scale Fuel-Coolant Interaction Experiments, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 1998-2009, 2006.
Moriyama, K., Nakamura, H., and Maruyama, Y., Analytical Tool Development for Coarse Break-Up of a Molten Jet in a Deep Water Pool, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 2010-2025, 2006.
Mularz, E. J. and Yuen, M. C., An Experimental Investigation of Radiative Properties of Aluminium Oxide Particles, J. Quant. Spectr. Radiat. Transfer, vol. 12, no. 11, pp. 1553-1568, 1972.
Petrov, V. A. and Chernyshev, A. P., Thermal-Radiation Properties of Zirconia when Heated by Laser Radiation up to Temperature of High-Rate Vaporization, High Temp., vol. 37, no. 1, pp. 58-66, 1999.
Pohlner, G., Vujic, Z., Bürger, M., and Lohnert, G., Simulation of Melt Jet Breakup and Debris Bed Formation in Water Pools with IKEJET/IKEMIX, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 2026-2048, 2006.
Rubtsov, N. A., Emelianov, A. A., and Ponomarev, N. N., Investigation of Fused Aluminium Oxide Absorption Index at High Temperatures, High Temp., vol. 22, no. 2, pp. 294-298, 1984 (in Russian).
Ruello, P., Becker, K. D., Ulrich, K., Desgranges, L., Petot, C., and Petot-Ervas, G., Thermal Variation of the Optical Absorption of UO2: Determination of the Small Polaron Self-Energy, J. Nucl. Mater., vol. 328, no. 1, pp. 46-54, 2004.
Theofanous, T. G., The Study of Steam Explosions in Nuclear Systems, Nucl. Eng. Des., vol. 155, no. 1-2, pp. 1-26, 1995.
Tseng, C. C. and Viskanta, R., On the Hypothesis of Thermal Phase Change, Int. Comm. Heat Mass Transfer, vol. 32, no. 10, pp. 1267-1272, 2005.
Wood, D. L. and Nassau, K., Refractive Index of Cubic Zirconia Stabilized with Yttria, Appl. Optics, vol. 21, no. 16, pp. 2978-2981, 1982.
References
- Abe, Y., Matsuo, E., Arai, T., Nariai, H., Chitose, K., Koyama, K., and Itoh, K., Fragmentation Behavior during Molten Material and Coolant Interactions, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 1668-1681, 2006.
- Akopov, F. A., Val’ano, G. E., Vorob’ev, A. Yu., Mineev, V. N., Petrov, V. A., Chernyshev, A. P., and Chernyshev, G. P., Thermal Radiative Properties of Ceramic of Cubic ZrO2 Stabilized with Y2O3 at High Temperatures, High Temp., vol. 39, no. 2, pp. 244-254, 2001.
- Alipchenkov, V. M., Dombrovsky, L. A., and Zaichik, L. I., The Growth and Stability of Vapor Film on the Surface of a Hot Spherical Particle, High Temp., vol. 40, no. 1, pp. 100-104, 2002 .
- Anderson, E. E., Radiative Heat Transfer in Molten UO2 Based on the Rosseland Diffusion Method, Nucl. Tech., vol. 30, pp. 65-70, 1976.
- Berthoud, G., Vapor Explosions, Ann. Rev. Fluid Mech., vol. 32, pp. 573-611, 2000.
- Bober, M., Singer, J., and Wagner, K., Determination of the Optical Constants of Liquid UO2 from Reflectivity Measurements, Proc. of 8th Symp. Thermophys. Prop., Gaithersburg, MD, vol. II, ASME, pp. 234-244, 1981.
- Bober, M., Singer, J., and Wagner, K., Bestimmung der Optischen Konstanten von Geschmolzenen Kernbrennstoffen, J. Nucl. Mater., vol. 124, pp. 120-128, 1984.
- Bürger, M., Particulate Debris Formation by Breakup of Melt Jets: Main Objectives and Solution Perspectives, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 1991-1997, 2006.
- Cabannes, F. and Billard, D., Measurement of Infrared Absorption of Some Oxides in Connection with the Radiative Transfer in Porous and Fibrous Materials, Int. J. Thermophys., vol. 8, no. 1, pp. 97-118, 1987.
- Corradini, M. L., Kim, B. J., and Oh, M. D., Vapor Explosion in Light Water Reactors: A Review of Theory and Modeling, Prog. Nucl. Energy, vol. 22, no. 1, pp. 1-117, 1988.
- Dinh, T. N., Material Property Effect in Steam Explosion Energetics: Revisited, Proc. of 12th Int. Topical Meeting Nucl. Reactor Therm. Hydraulics (NURETH-12), Pittsburg, PA, Sept. 30-Oct. 4, Amer. Nucl. Soc., Paper No. 150, 2007.
- Dinh, T. N., Bui, V. A., Nourgaliev, R. R., Green, J. A., and Sehgal, B. R., Experimental and Analytical Studies of Melt Jet-Coolant Interactions: A Synthesis, Nucl. Eng. Des., vol. 189, no. 1-3, pp. 299-327, 1999a.
- Dinh, T. N., Dinh, A. T., Nourgaliev, R. R., and Sehgal, B. R., Investigation of Film Boiling Thermal Hydraulics Under FCI Conditions: Results of Analyses and Numerical Study, Nucl. Eng. Des., vol. 189, no. 1-3, 251-272, 1999b.
- Dombrovsky, L. A., Possibility of Determining the Disperse Composition of a Two-Phase Flow from the Small-Angle Light Scattering, High Temp., vol. 20, no. 3, pp. 472-479, 1982.
- Dombrovsky, L. A., Radiation Heat Transfer in Disperse Systems, Begell House, New York and Redding, CT, 1996.
- Dombrovsky, L. A., Radiation Heat Transfer from a Spherical Particle via Vapor Shell to the Surrounding Liquid, High Temp., vol. 37, no. 6, pp. 912-919, 1999.
- Dombrovsky, L. A., Radiation Heat Transfer from a Hot Particle to Ambient Water through the Vapor Layer, Int. J. Heat Mass Transfer, vol. 43, no. 13, pp. 2405-2414, 2000.
- Dombrovsky, L. A., A Spectral Model of Absorption and Scattering of Thermal Radiation by Droplets of Diesel Fuel, High Temp., vol. 40, no. 2, pp. 242-248, 2002.
- Dombrovsky, L. A., Radiation Transfer through a Vapour Gap under Conditions of Film Boiling of Liquid, High Temp., vol. 41, no. 6, pp. 819-824, 2003.
- Dombrovsky, L. A., Thermal Radiation of Nonisothermal Particles in Combined Heat Transfer Problems (dedication lecture), Proc. of 5th Int. Symp. Radiat. Transfer, Bodrum, Turkey, June 17-22, Begell House, 2007a.
- Dombrovsky, L. A., An Estimate of Stability of Large Solidifying Droplets in Fuel-Coolant Interaction, Int. J. Heat Mass Transfer, vol. 50, no. 19-20, pp. 3832-3836, 2007b.
- Dombrovsky, L. A., Davydov, M. V., and Kudinov, P., Thermal Radiation Modeling in Numerical Simulation of Melt-Coolant Interaction, Comput. Thermal Sci., vol. 1, no. 1, pp. 1-35, 2009.
- Dombrovsky, L. A. and Dinh, T. N., The Effect of Thermal Radiation on the Solidification Dynamics of Metal Oxide Melt Droplets, Nucl. Eng. Des., vol. 238, no. 6, pp. 1421-1429, 2008.
- Dombrovsky, L. A. and Lipiński, W., Transient Temperature and Thermal Stress Profiles in Semi-Transparent Particles Under High-Flux Irradiation, Int. J. Heat Mass Transfer, vol. 50, no. 11-12, pp. 2117-2123, 2007.
- Dombrovsky, L. A. and Sazhin, S. S., A Parabolic Temperature Profile Model for Heating of Droplets, ASME J. Heat Transfer, vol. 125, no. 3, pp. 535-537, 2003a.
- Dombrovsky, L. A. and Sazhin, S. S., A Simplified Nonisothermal Model for Droplet Heating and Evaporation, Int. Comm. Heat Mass Transfer, vol. 30, no. 6, pp. 787-796, 2003b.
- Dombrovsky, L. A., Tagne, H. K., Baillis, D., and Gremillard, L., Near-Infrared Radiative Properties of Porous Zirconia Ceramics, Infrared Phys. Tech., vol. 51, no. 1, pp. 44-53, 2007.
- Dombrovsky, L. A. and Zaichik, L. I., The Dynamics of Vapor Void Under Conditions of Thermal Interaction of a Hot Spherical Particle with Ambient Water, High Temp., vol. 38, no. 6, pp. 938-947, 2000.
- Fink, J. K., Thermophysical Properties of Uranium Dioxide (Review), J. Nucl. Mater., vol. 279, no. 1, pp. 1-18, 2000.
- Fletcher, D. F., The Particle Size Distribution of Solidified Melt Debris from Molten Fuel-Coolant Interaction Experiments, Nucl. Eng. Des., vol. 105, no. 3, pp. 313-319, 1988.
- Fletcher, D. F., Steam Explosion Triggering: A Review of Theoretical and Experimental Investigations, Nucl. Eng. Des., vol. 155, no. 1-2, pp. 27-36, 1995.
- Fletcher, D. F., Radiation Absorption During Premixing, Nucl. Eng. Des., vol. 189, no. 1-3, pp. 435-440, 1999.
- Fletcher, D. F. and Anderson, R. P., A Review of Pressure-Induced Propagation Models of the Vapor Explosion Process, Prog. Nucl. Energy, vol. 23, no. 2, pp. 137-179, 1990.
- Harding, J. H., Martin, D. G., and Potter, P. E., Thermophysical and Thermochemical Properties of Fast Reactor Materials, Commiss. Eur. Commun. Report EUR 12402 EN, 1989.
- Huhtiniemi, I., Magallon, D., and Hohmann, H., Results of Recent KROTOS FCI Tests: Alumina Versus Corium Melts, Nucl. Eng. Des., vol. 189, no. 1-3, pp. 379-389, 1999.
- Lingart, Yu. K., Petrov, V. A., and Tikhonova, N. A., Optical Properties of Synthetic Sapphire at High Temperatures. II. Properties of Monocrystal in Opacity Region and Melt Properties, High Temp., vol. 20, no. 6, pp. 1085-1092, 1982.
- Magallon, D., Characteristics of Corium Debris Bed Generated in Large-Scale Fuel-Coolant Interaction Experiments, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 1998-2009, 2006.
- Moriyama, K., Nakamura, H., and Maruyama, Y., Analytical Tool Development for Coarse Break-Up of a Molten Jet in a Deep Water Pool, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 2010-2025, 2006.
- Mularz, E. J. and Yuen, M. C., An Experimental Investigation of Radiative Properties of Aluminium Oxide Particles, J. Quant. Spectr. Radiat. Transfer, vol. 12, no. 11, pp. 1553-1568, 1972.
- Petrov, V. A. and Chernyshev, A. P., Thermal-Radiation Properties of Zirconia when Heated by Laser Radiation up to Temperature of High-Rate Vaporization, High Temp., vol. 37, no. 1, pp. 58-66, 1999.
- Pohlner, G., Vujic, Z., Bürger, M., and Lohnert, G., Simulation of Melt Jet Breakup and Debris Bed Formation in Water Pools with IKEJET/IKEMIX, Nucl. Eng. Des., vol. 236, no. 19-21, pp. 2026-2048, 2006.
- Rubtsov, N. A., Emelianov, A. A., and Ponomarev, N. N., Investigation of Fused Aluminium Oxide Absorption Index at High Temperatures, High Temp., vol. 22, no. 2, pp. 294-298, 1984 (in Russian).
- Ruello, P., Becker, K. D., Ulrich, K., Desgranges, L., Petot, C., and Petot-Ervas, G., Thermal Variation of the Optical Absorption of UO2: Determination of the Small Polaron Self-Energy, J. Nucl. Mater., vol. 328, no. 1, pp. 46-54, 2004.
- Theofanous, T. G., The Study of Steam Explosions in Nuclear Systems, Nucl. Eng. Des., vol. 155, no. 1-2, pp. 1-26, 1995.
- Tseng, C. C. and Viskanta, R., On the Hypothesis of Thermal Phase Change, Int. Comm. Heat Mass Transfer, vol. 32, no. 10, pp. 1267-1272, 2005.
- Wood, D. L. and Nassau, K., Refractive Index of Cubic Zirconia Stabilized with Yttria, Appl. Optics, vol. 21, no. 16, pp. 2978-2981, 1982.